Research
Cell cycle regulation
In order to proliferate, cells need to replicate their material, segregate it and divide. The coordination of these events is what is called cell cycle control. It depends on a network of key regulatory enzymes including kinases and phosphatases that are themselves regulated in abundance, in activity and in localization. Defects in the mechanisms of cell cycle control can give rise to cancer.
The Drosophila model
The molecular network that controls the cell division cycle is conserved between species. We use the fruitfly Drosophila as a model. The genetic tools it provides are extremely well developed and powerful. We can examine cell division in several tissues and in Drosophila cells in culture. Mutated and fluorescently tagged proteins can be expressed in tissues of interest and the expression of endogenous proteins can be silenced by RNA interference.

Drosophila melanogaster
female (left) and male (right)
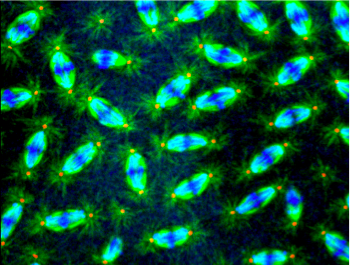
Nuclear divisions in a Drosophila early embryo at the syncytial stage. This embryo is from a mother mutated in genes encoding mitotic kinases of interest. Examples of mitotic defects are pointed by arrows.


The Greatwall - PP2A axis
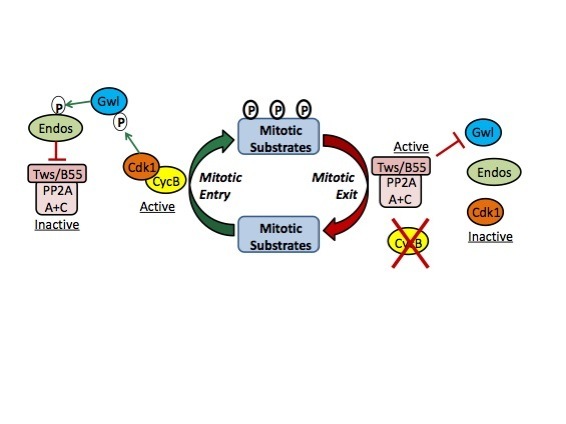
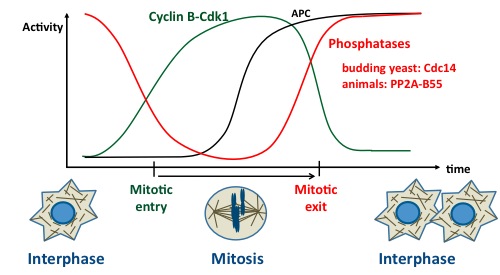
Mitosis is triggered mainly by the activation of Cyclin B-Cdk1, which promotes nuclear envelope breakdown, chromosome condensation, spindle assembly and other events. Once chromosomes have been segregated, the newly forming cells need to return into interphase. This process requires the inactivation of Cyclin B-Cdk1 which occurs largely via the ubiquitination of cyclin B by the Anaphase Promoting Complex (APC) and its subsequent degradation by the proteasome. Meanwhile, several substrates of Cyclin B-Cdk1 must be dephosphorylated. The protein phosphatase 2A (PP2A) in complex with B55 regulatory subunits (Tws in Drosophila) plays an important role in this process (see model on right).
But how is PP2A-Tws regulated? The key to solve this enigma came from the discovery of the Greatwall (Gwl) kinase in Drosophila. This kinase is required for mitosis and meiosis in vivo. Seminal work in frog extracts, in flies and in human cells has established that Gwl phosphorylates the protein endosulfine (and Arpp19 in vertebrates) which becomes an inhibitor of PP2A-B55. At mitotic entry, Gwl activation leads to PP2A-B55 inactivation. Conversely, at mitotic exit, this mechanism must be turned off to allow reactivation of PP2A-B55 (see model on right).
The Gwl-PP2A axis in cell cycle control
How are Gwl and Endos themselves regulated? We are interested in this question. We have found that Gwl is subjected to a strict spatial regulation during the cell cycle. From a nuclear localization in interphase, it becomes cytoplasmic and excluded from the nucleus in prophase, while the nuclear envelope still acts as a diffusion barrier (see below and Movies section).
By what mechanisms does Gwl change localization in the cell cycle? We have found that the Polo and Cdk1 kinases promote the relocalization of Gwl to the cytoplasm, where PP2A-Tws/B55 is concentrated.
Because Cyclin B-Cdk1 activation occurs primarily in the nucleus, the nuclear localization of Gwl could help its activation by Cyclin B-Cdk1. Moreover, as PP2A-B55 is primarily cytoplasmic, the nuclear localization of Cyclin B-Cdk1 and Gwl could protect them from dephosphorylation by PP2A-B55 during their activation. The sudden export of activated Gwl to the cytoplasm could be required for efficient inhibition of PP2A-B55.
We want to discover other molecular mechanisms that regulate this pathway.
We also want to identify the phosphorylated proteins which must be protected from PP2A-B55 at mitotic entry, and those which must be dephosphorylated by PP2A-B55 at mitotic exit.
More generally, we are interested in the importance of spatial coordination of the main cell cycle regulators relative to each other and relative to substrates that act as effectors during cell division.
The Polo Kinase
Discovered in Drosophila, the Polo kinase is another major regulator of mitosis and cytokinesis. It is required for centrosome maturation, kinetochore function, bipolar spindle assembly, cytokinesis and centromere maintenance. Discovered in Drosophila, this protein is conserved from yeast to humans.
Polo-like kinase 1 (Plk1) is currently targeted by anti-cancer agents in development. Although it is required for the division of all cells, several cancer cells depend on a higher level of Plk1 activity than healthy cells for their division and survival.
Polo kinase is tightly regulated in localization. Its C-terminal Polo-Box Domain (PBD) mediates protein interactions with targets and regulators. The PBD allows Polo to localize to discrete structures during mitosis and cytokinesis, where its kinase domain (KD) phosphorylates numerous substrate proteins to modify their activities (see below and Movies section).
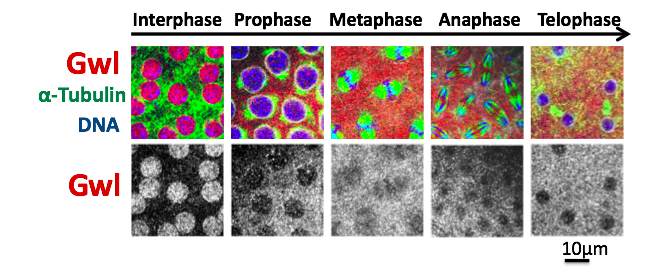
Immunofluorescence in fixed Drosophila syncytial embryos.
Gwl changes from a nuclear to a cytoplasmic localization in prophase.
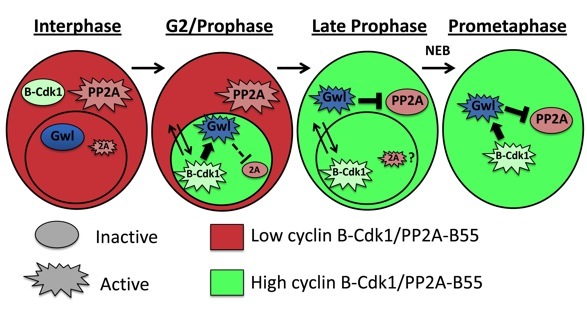
Model for the spatial regulation of Gwl in the cell cycle.
Oscillations of enzymatic activities control cell division
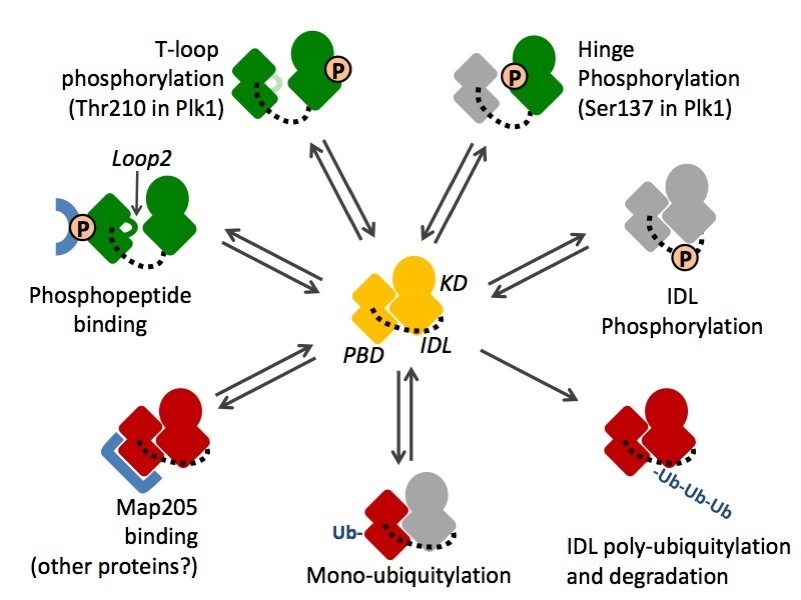
Several mechanisms contribute to regulate Polo activities during the cell cycle. Perturbations of the kinase domain (KD) or of the Polo-Box Domain (PBD) can activate or inhibit their functions. In addition, perturbations of one domain can modify the activities of the other domain by allosteric effects. IDL: inter-domain linker. Green: activated domain; Red: inhibited domain; Grey: unknown effect.
How are these multiple levels of regulation integrated in time and space to allow Polo kinase to fulfill its multiple functions in the cell cycle?
Can we develop molecules that specifically modulate Polo kinase activities? Could some of them be used in cancer treatments?
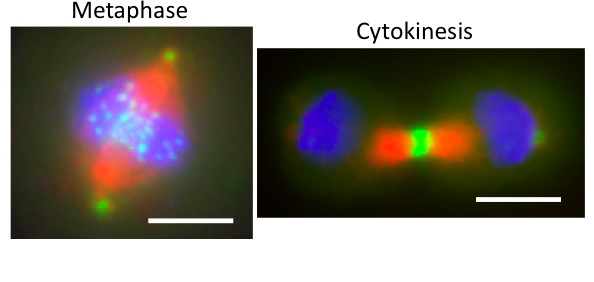
Drosophila cells in culture
expressing Polo-GFP (green)
and stained for Tubulin (red)
and DNA (blue). Polo localizes
to centrosomes and kinetochores
in metaphase, and at the midbody
in cytokinesis. Scale bars: 5 uM.
Therefore, the two domains of Polo collaborate. However, they are also capable of reciprocal inhibition by an intramolecular interaction. The control of multiple cell cycle events by the Polo kinase is enabled by multiple levels of regulation of this protein’s functions.